Measuring dissolution profiles of single controlled-release drug pellets
Heran C. Bhakta, Jessica M. Lin, and William H. Grover, Scientific Reports 10, 19734 (2020) .
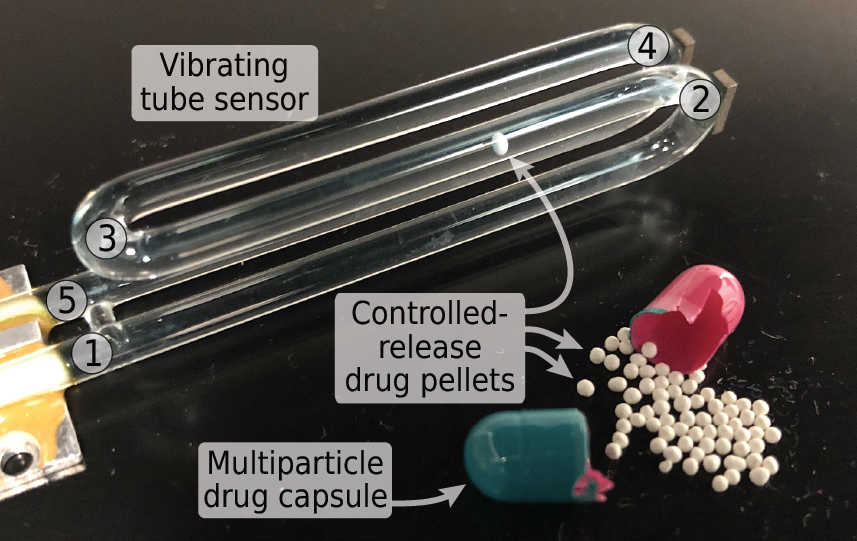
Many solid-dose oral drug products are engineered to release their active ingredients into the body at a certain rate. Techniques for measuring the dissolution or degradation of a drug product in vitro play a crucial role in predicting how a drug product will perform in vivo. However, existing techniques are often labor-intensive, time-consuming, irreproducible, require specialized analytical equipment, and provide only “snapshots” of drug dissolution every few minutes. These limitations make it difficult for pharmaceutical companies to obtain full dissolution profiles for drug products in a variety of different conditions, as recommended by the US Food and Drug Administration. Additionally, for drug dosage forms containing multiple controlled-release pellets, particles, beads, granules, etc. in a single capsule or tablet, measurements of the dissolution of the entire multi-particle capsule or tablet are incapable of detecting pellet-to-pellet variations in controlled release behavior. In this work, we demonstrate a simple and fully-automated technique for obtaining dissolution profiles from single controlled-release pellets. We accomplished this by inverting the drug dissolution problem: instead of measuring the increase in the concentration of drug compounds in the solution during dissolution (as is commonly done), we monitor the decrease in the buoyant mass of the solid controlled-release pellet as it dissolves. We weigh single controlled-release pellets in fluid using a vibrating tube sensor, a piece of glass tubing bent into a tuning-fork shape and filled with any desired fluid. An electronic circuit keeps the glass tube vibrating at its resonance frequency, which is inversely proportional to the mass of the tube and its contents. When a pellet flows through the tube, the resonance frequency briefly changes by an amount that is inversely proportional to the buoyant mass of the pellet. By passing the pellet back-and-forth through the vibrating tube sensor, we can monitor its mass as it degrades or dissolves, with high temporal resolution (measurements every few seconds) and mass resolution (700 nanogram resolution). As a proof-of-concept, we used this technique to measure the single-pellet dissolution profiles of several commercial controlled-release proton pump inhibitors in simulated stomach and intestinal contents, as well as comparing name-brand and generic formulations of the same drug. In each case, vibrating tube sensor data revealed significantly different dissolution profiles for the different drugs, and in some cases our method also revealed differences between different pellets from the same drug product. By measuring any controlled-release pellets, particles, beads, or granules in any physiologically-relevant environment in a fully-automated fashion, this method can augment and potentially replace current dissolution tests and support product development and quality assurance in the pharmaceutical industry.