Our work on obtaining dissolution profiles from single controlled-release drug particles using vibrating tube sensors published in Scientific Reports
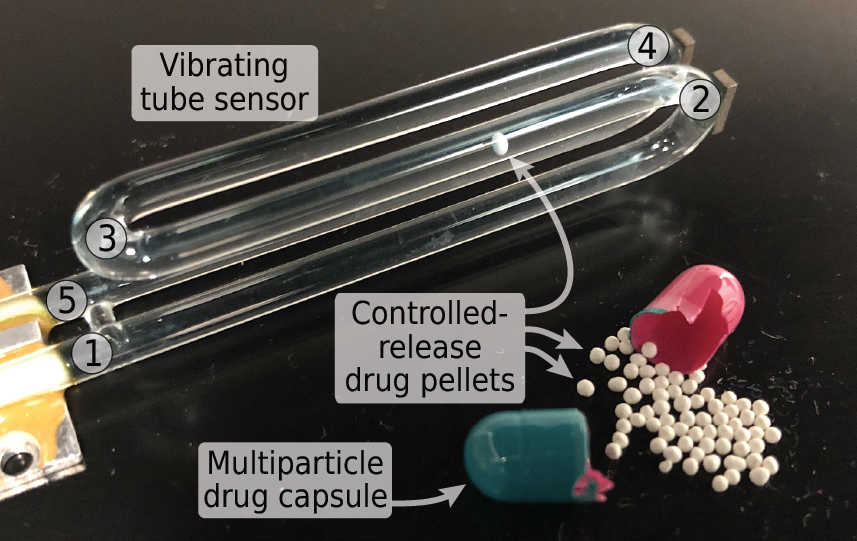
Heran Bhakta and Jessica Lin’s paper “Measuring dissolution profiles of single controlled-release drug pellets” was published in Scientific Reports.
Heran and Jessica show that a simple and inexpensive vibrating tube sensor can measure the dissolution of single microgram-sized controlled-release pellets in physiologically relevant fluids with nanogram-scale resolution. Their technique addresses many of the shortcomings of existing USP testing methods, requires no additional analytical instrumentation like UV-VIS or HPLC, and is suitable for both fast-dissolving and slow-dissolving formulations. And by obtaining dissolution profiles for single pellets instead of populations of pellets, their technique is capable of measuring pellet-to-pellet variations in dissolution behavior that are much more difficult to measure using existing methods. Using this technique, we observed significant variations in single-pellet dissolution profiles, not only between different types of drugs in different physiological conditions, but also between generic and name-brand formulations of the same drug, and even between different pellets from the exact same capsule.
This technique provides pharmaceutical researchers and producers with a simple, low-cost, and fully automated tool for obtaining single-pellet dissolution profiles from any drug in any desired fluid. This capability should be powerful in a variety of different scenarios. For example, measurements of the dissolution behavior of pellets from each production batch can provide valuable quality assurance data and illuminate possible production defects before the product reaches consumers. Even within a single batch, single pellet dissolution profiles provide information about the consistency of the pellet manufacturing process. And as a gravimetric (mass-based) method, this technique places no constraints on the chemical or physical composition of the fluid surrounding the pellet, meaning that pharmaceutical developers are free to measure pellet dissolution in any physiologically-relevant fluid without fear that the fluid will interfere with the measurement process. For these reasons, vibrating tube sensors should help facilitate the development of better controlled-release pharmaceuticals with better patient outcomes.